H C C H Lewis Structure
planetorganic
Nov 18, 2025 · 11 min read
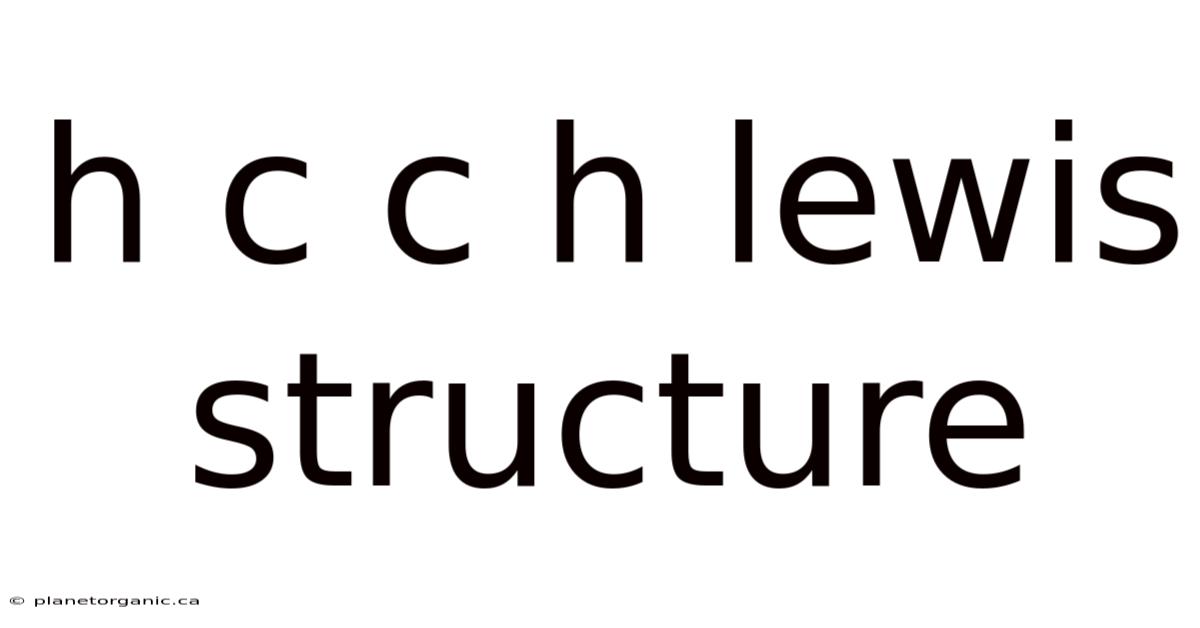
Table of Contents
HCCH, or diacetylene, is a fascinating molecule in the realm of organic chemistry. Understanding its structure, particularly through the lens of its Lewis structure, provides valuable insights into its bonding, reactivity, and overall properties. This article delves into the intricate details of the HCCH Lewis structure, guiding you through the process of constructing it, explaining the underlying principles, and exploring its significance in chemistry.
Understanding the Basics: What is a Lewis Structure?
A Lewis structure, also known as a Lewis dot diagram, is a visual representation of a molecule's bonding and non-bonding electrons. It uses dots to represent valence electrons (electrons in the outermost shell of an atom) and lines to represent covalent bonds (shared electrons between atoms). Lewis structures are essential tools for:
- Predicting molecular geometry: The arrangement of atoms in space.
- Understanding bond polarity: Whether a bond is equally shared or leaning towards one atom.
- Determining reactivity: Identifying sites prone to chemical reactions.
Why Diacetylene (HCCH) Matters
Diacetylene is a linear molecule comprised of two carbon atoms and two hydrogen atoms. Its defining feature is the presence of two triple bonds between the carbon atoms and a single bond between each carbon and hydrogen atom. This unique arrangement makes it an important molecule in astrochemistry, materials science, and theoretical chemistry.
- Astrochemistry: Diacetylene has been detected in interstellar space and planetary atmospheres, contributing to our understanding of the chemical processes occurring in these environments.
- Materials Science: It serves as a building block for synthesizing novel carbon-rich materials with unique electrical and optical properties.
- Theoretical Chemistry: Its relatively simple structure makes it an ideal model system for studying the electronic structure and bonding in alkynes.
Constructing the HCCH Lewis Structure: A Step-by-Step Guide
Let's embark on the process of constructing the Lewis structure for diacetylene (HCCH). We'll follow a methodical approach, ensuring we account for all valence electrons and adhere to the octet rule (or duet rule for hydrogen).
Step 1: Determine the Total Number of Valence Electrons
First, we need to identify the number of valence electrons each atom contributes:
- Hydrogen (H) has 1 valence electron.
- Carbon (C) has 4 valence electrons.
In HCCH, we have two hydrogen atoms and two carbon atoms. Therefore, the total number of valence electrons is:
(2 H atoms * 1 valence electron/H atom) + (2 C atoms * 4 valence electrons/C atom) = 2 + 8 = 10 valence electrons
Step 2: Draw the Skeletal Structure
Next, we arrange the atoms in a plausible order. Since diacetylene is a linear molecule, the skeletal structure is:
H - C - C - H
Step 3: Place Single Bonds Between Atoms
Connect the atoms with single bonds, representing the sharing of two electrons:
H - C C - H
Each single bond accounts for two electrons. So far, we've used 3 single bonds * 2 electrons/bond = 6 electrons.
Step 4: Distribute Remaining Electrons as Lone Pairs
We have 10 total valence electrons and have used 6 electrons for single bonds. This leaves us with 10 - 6 = 4 electrons. These remaining electrons need to be distributed as lone pairs around the carbon atoms to satisfy the octet rule.
However, simply adding lone pairs won't achieve a stable octet for each carbon. We need to form multiple bonds.
Step 5: Form Multiple Bonds to Satisfy the Octet Rule
Each carbon atom currently has only two shared electrons (one from the bond with hydrogen and one from the bond with the adjacent carbon). To achieve an octet (8 electrons) around each carbon, we need to form triple bonds between the carbon atoms.
Replace the single bond between the carbon atoms with a triple bond:
H - C ≡ C - H
Each triple bond consists of three pairs of shared electrons (6 electrons). Now, let's check the electron count around each atom:
- Each hydrogen atom shares 2 electrons (satisfying the duet rule).
- Each carbon atom shares 8 electrons (1 from the C-H bond and 3 from each C≡C bond, satisfying the octet rule).
Step 6: Verify the Lewis Structure
Ensure that all atoms have a stable electron configuration (duet for hydrogen, octet for carbon) and that the total number of valence electrons used matches the initial calculation.
In the HCCH Lewis structure:
- Both hydrogen atoms have 2 electrons.
- Both carbon atoms have 8 electrons.
- We have used a total of 10 valence electrons (2 from each C-H single bond and 6 from the C≡C triple bond).
Therefore, the HCCH Lewis structure is:
H - C ≡ C - H
Understanding the Bonding in Diacetylene
The HCCH Lewis structure reveals a crucial aspect of its bonding: the presence of a triple bond between the two carbon atoms. This triple bond is composed of one sigma (σ) bond and two pi (π) bonds.
- Sigma (σ) Bond: A sigma bond is formed by the head-on overlap of atomic orbitals, resulting in electron density concentrated along the internuclear axis.
- Pi (π) Bonds: Pi bonds are formed by the sideways overlap of p orbitals, resulting in electron density above and below the internuclear axis.
The combination of one sigma bond and two pi bonds in the triple bond makes it a very strong bond, contributing to the relative stability of diacetylene. However, the presence of these pi bonds also makes the molecule reactive, as the pi electrons are more easily accessible for chemical reactions compared to sigma electrons.
Resonance Structures: Are They Relevant for HCCH?
Resonance structures are different Lewis structures for the same molecule that differ only in the distribution of electrons. They are used when a single Lewis structure cannot accurately represent the bonding in a molecule. While resonance is important for many molecules, it's not a significant factor for diacetylene (HCCH). The Lewis structure we derived (H-C≡C-H) is a good and accurate representation of the molecule's electron distribution. There aren't other significantly contributing resonance structures.
Formal Charge Considerations
Formal charge is a concept used to assess the distribution of electrons in a Lewis structure. It helps determine if a particular Lewis structure is more or less likely to represent the actual molecule. The formal charge of an atom in a Lewis structure is calculated as:
Formal Charge = (Valence Electrons) - (Non-bonding Electrons) - (1/2 * Bonding Electrons)
Let's calculate the formal charges for each atom in HCCH:
- Hydrogen (H): 1 - 0 - (1/2 * 2) = 0
- Carbon (C): 4 - 0 - (1/2 * 8) = 0
All atoms in the HCCH Lewis structure have a formal charge of zero. This indicates that the Lewis structure accurately represents the electron distribution in the molecule and is a stable configuration.
Beyond the Lewis Structure: Molecular Orbital Theory
While the Lewis structure provides a valuable foundation for understanding bonding, Molecular Orbital (MO) theory offers a more sophisticated and accurate description of electronic structure. MO theory considers the interaction of atomic orbitals to form molecular orbitals that extend over the entire molecule.
In the case of diacetylene, MO theory predicts a complex array of sigma and pi molecular orbitals. The highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) are particularly important because they determine the molecule's reactivity and electronic transitions. The triple bonds result in a high electron density and unique reactivity patterns compared to molecules with only single or double bonds.
Spectroscopic Properties and the HCCH Lewis Structure
The Lewis structure helps us understand the spectroscopic properties of diacetylene. Spectroscopic techniques, such as infrared (IR) spectroscopy and Raman spectroscopy, probe the vibrational modes of molecules. The presence of the C≡C triple bonds in diacetylene results in characteristic vibrational frequencies that can be observed in its IR and Raman spectra. These vibrational modes provide experimental evidence supporting the presence of the triple bonds predicted by the Lewis structure.
Furthermore, electronic transitions between different molecular orbitals can be studied using UV-Vis spectroscopy. The HCCH Lewis structure helps us understand the types of electronic transitions that are possible and predict the wavelengths at which these transitions will occur.
Chemical Reactivity and the Lewis Structure
The HCCH Lewis structure provides insights into the chemical reactivity of diacetylene. The triple bonds are electron-rich and susceptible to attack by electrophiles (electron-seeking species). Some common reactions of diacetylene include:
- Addition Reactions: Diacetylene can undergo addition reactions with reagents such as hydrogen, halogens, and acids. These reactions break the pi bonds in the triple bond and form new sigma bonds.
- Polymerization: Diacetylene can polymerize to form long chains of carbon atoms linked by alternating single and triple bonds. These polymers can have interesting electrical and optical properties.
- Cycloaddition Reactions: Diacetylene can participate in cycloaddition reactions, such as the Diels-Alder reaction, to form cyclic compounds.
The Lewis structure helps us understand the mechanism of these reactions by identifying the sites of highest electron density and predicting the products that will be formed.
HCCH in Astrochemistry: A Cosmic Building Block
As mentioned earlier, diacetylene has been detected in various astronomical environments, including interstellar clouds, circumstellar disks, and planetary atmospheres. Its presence in these environments suggests that it plays a role in the formation of more complex organic molecules, which are the building blocks of life.
The formation of diacetylene in space is believed to occur through a series of gas-phase reactions involving simpler molecules such as acetylene (C2H2) and ethynyl radicals (C2H). The extreme conditions in space, such as low temperatures and high radiation levels, can influence the rates and pathways of these reactions.
The detection of diacetylene in space provides valuable information about the chemical composition and evolution of these environments. It also raises intriguing questions about the origin of life and the possibility of extraterrestrial life.
HCCH in Materials Science: A Versatile Precursor
Diacetylene and its derivatives are used in materials science as precursors for synthesizing novel carbon-rich materials with unique properties. One example is the synthesis of polydiacetylenes (PDAs). PDAs are polymers with a conjugated backbone of alternating single and triple bonds. They exhibit interesting optical properties, such as thermochromism (color change with temperature) and mechanochromism (color change with mechanical stress).
These properties make PDAs attractive for applications such as:
- Sensors: PDAs can be used to detect changes in temperature, pressure, or chemical environment.
- Optical Devices: PDAs can be used in optical switches, waveguides, and other optical devices.
- Coatings: PDAs can be used as protective coatings for various materials.
The ability to tune the properties of PDAs by modifying the substituents attached to the diacetylene monomer makes them a versatile platform for developing new materials with specific applications.
Common Mistakes When Drawing the HCCH Lewis Structure
While the process of drawing the HCCH Lewis structure is relatively straightforward, there are some common mistakes that students and beginners often make:
- Incorrect Valence Electron Count: Forgetting to correctly calculate the total number of valence electrons is a frequent error. Always double-check the number of valence electrons for each atom and ensure the total is accurate.
- Failure to Satisfy the Octet Rule: Not ensuring that each carbon atom has an octet of electrons (8 electrons) is another common mistake. This often leads to incorrect bonding arrangements.
- Incorrect Placement of Bonds: Placing single bonds instead of triple bonds between the carbon atoms leads to an unstable structure and an incorrect representation of the molecule.
- Adding Lone Pairs to Carbon: Carbon atoms in HCCH do not have lone pairs in the correct Lewis structure. Adding lone pairs will result in a formal charge and an unstable configuration.
- Ignoring Formal Charge: While HCCH has a simple Lewis structure where formal charges are zero, in more complex molecules, ignoring formal charges can lead to incorrect Lewis structures.
Practice Problems and Exercises
To solidify your understanding of the HCCH Lewis structure and Lewis structures in general, try working through these practice problems:
- Draw the Lewis structure for acetylene (C2H2). How does it compare to the Lewis structure of diacetylene (HCCH)?
- Draw the Lewis structure for carbon monoxide (CO). What is the bond order between carbon and oxygen?
- Draw the Lewis structure for nitrogen gas (N2). What type of bond exists between the nitrogen atoms?
- Draw the Lewis structure for hydrogen cyanide (HCN). Calculate the formal charge on each atom.
- Draw the Lewis structure for carbon dioxide (CO2). Are there any resonance structures?
By practicing these problems, you'll develop your skills in constructing Lewis structures and understanding the bonding in molecules.
Conclusion: The Power of the Lewis Structure
The Lewis structure of diacetylene (HCCH) is a simple yet powerful tool for understanding the molecule's bonding, reactivity, and properties. By following a step-by-step approach, we can accurately represent the distribution of electrons and gain insights into the molecule's electronic structure. While more advanced theories like Molecular Orbital theory provide a more complete picture, the Lewis structure remains an essential foundation for understanding chemical bonding. From its role as a cosmic building block in astrochemistry to its application as a versatile precursor in materials science, diacetylene continues to be a fascinating molecule that drives scientific discovery. Understanding its Lewis structure is the first step in unraveling its secrets.
Latest Posts
Latest Posts
-
Unilevers New Global Strategy Competing Through Sustainability
Nov 18, 2025
-
Rn 3 0 Clinical Judgment Practice 3
Nov 18, 2025
-
Population Genetics And Evolution Lab Answer Key
Nov 18, 2025
-
Ammonium Chloride Major Species Present When Dissolved In Water
Nov 18, 2025
-
Increases The Angle Of A Joint
Nov 18, 2025
Related Post
Thank you for visiting our website which covers about H C C H Lewis Structure . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.