Draw The Product Of The Hydration Of 2 Butene
planetorganic
Nov 28, 2025 · 9 min read
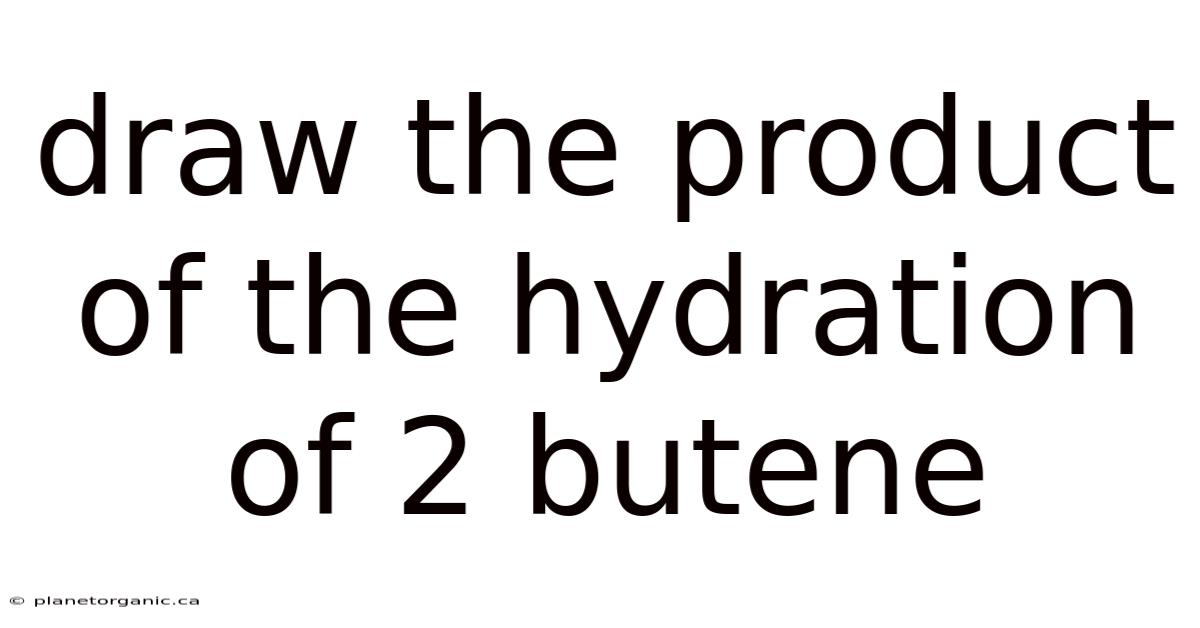
Table of Contents
The hydration of 2-butene is a fundamental organic chemistry reaction that involves the addition of water (H₂O) to an alkene, specifically 2-butene, to produce an alcohol. This reaction is an example of electrophilic addition and is typically carried out in the presence of an acid catalyst, such as sulfuric acid (H₂SO₄) or phosphoric acid (H₃PO₄). Understanding the mechanism and product formation of this reaction is crucial for students and professionals in chemistry.
Introduction to Alkene Hydration
Alkene hydration is a chemical process where a water molecule is added across the double bond of an alkene. Alkenes, characterized by the presence of at least one carbon-carbon double bond, are unsaturated hydrocarbons that are more reactive than their alkane counterparts. The hydration reaction converts an alkene into an alcohol, where a hydroxyl group (-OH) and a hydrogen atom are added to the carbon atoms of the double bond.
2-Butene is a four-carbon alkene with the double bond located between the second and third carbon atoms. It exists as two isomers: cis-2-butene and trans-2-butene. The hydration of 2-butene follows Markovnikov's rule, which states that in the addition of a protic acid HX to an alkene, the hydrogen atom adds to the carbon atom with the greater number of hydrogen atoms, and the X group adds to the carbon atom with the fewer hydrogen atoms.
Reaction Mechanism of 2-Butene Hydration
The hydration of 2-butene proceeds through a three-step mechanism:
-
Protonation:
- The reaction begins with the protonation of the double bond of 2-butene by an acid catalyst (e.g., H₂SO₄). The π electrons of the double bond attack a proton (H⁺) from the acid, forming a carbocation intermediate.
- In the case of 2-butene, the proton can add to either the second or third carbon atom. Since both carbon atoms are equally substituted, the carbocation formed is a secondary carbocation (2° carbocation). This is a crucial point because secondary carbocations are more stable than primary carbocations but less stable than tertiary carbocations.
-
Nucleophilic Attack:
- The carbocation intermediate is highly electron-deficient and thus susceptible to nucleophilic attack. A water molecule (H₂O), acting as a nucleophile, attacks the carbocation, forming an oxonium ion.
-
Deprotonation:
- The oxonium ion is unstable and quickly deprotonates to form the alcohol product. Another water molecule acts as a base, accepting a proton from the oxonium ion, regenerating the acid catalyst (H⁺) and yielding the final alcohol product.
Detailed Step-by-Step Mechanism
To provide a clearer understanding, let's break down the mechanism with specific chemical structures and explanations:
-
Protonation of 2-Butene:
- cis-2-Butene or trans-2-Butene reacts with the acid catalyst (H₂SO₄). The double bond accepts a proton (H⁺), leading to the formation of a secondary carbocation.
CH₃CH=CHCH₃ + H⁺ → CH₃CH⁺CH₂CH₃ -
Nucleophilic Attack by Water:
- The carbocation intermediate reacts with a water molecule. The oxygen atom in water has lone pairs of electrons that can attack the positively charged carbon atom of the carbocation, forming an oxonium ion.
CH₃CH⁺CH₂CH₃ + H₂O → CH₃CH(OH₂)⁺CH₂CH₃ -
Deprotonation to Form the Alcohol:
- The oxonium ion is deprotonated by another water molecule, regenerating the acid catalyst and forming the final alcohol product, 2-butanol.
CH₃CH(OH₂)⁺CH₂CH₃ + H₂O → CH₃CH(OH)CH₂CH₃ + H₃O⁺- The final product is 2-butanol.
Product Prediction and Markovnikov's Rule
Markovnikov's rule is crucial in predicting the product of alkene hydration. In the hydration of 2-butene, the double bond is between two secondary carbon atoms. When the proton adds to one of these carbon atoms, it forms a secondary carbocation. Since both carbon atoms are equally substituted, the water molecule can add to either carbon, resulting in the same product: 2-butanol.
Stereochemistry of the Product
The hydration of 2-butene can lead to the formation of a chiral center at the second carbon atom of 2-butanol. Therefore, the product is a racemic mixture of both enantiomers: (R)-2-butanol and (S)-2-butanol.
- (R)-2-Butanol: The (R) enantiomer has the substituents arranged in a clockwise direction around the chiral center according to the Cahn-Ingold-Prelog (CIP) priority rules.
- (S)-2-Butanol: The (S) enantiomer has the substituents arranged in a counterclockwise direction around the chiral center.
Since the carbocation intermediate is sp² hybridized and planar, the water molecule can attack from either side of the plane with equal probability, resulting in the formation of a racemic mixture.
Factors Affecting the Hydration Reaction
Several factors can influence the rate and yield of the hydration reaction:
- Acid Concentration: Higher concentrations of the acid catalyst typically increase the reaction rate by facilitating the protonation step.
- Temperature: Higher temperatures generally increase the reaction rate, but excessively high temperatures can lead to side reactions, such as alkene isomerization or polymerization.
- Solvent: The choice of solvent can affect the reaction rate. Polar solvents, such as water or alcohols, are generally used to facilitate the hydration process.
- Steric Hindrance: Sterically hindered alkenes may react more slowly due to the difficulty of the protonation step.
Side Reactions and Byproducts
While the main product of 2-butene hydration is 2-butanol, side reactions can occur, leading to the formation of byproducts:
- Alkene Isomerization: Under acidic conditions, 2-butene can undergo isomerization to form 1-butene. This can occur if the carbocation intermediate undergoes a hydride shift.
- Polymerization: Alkenes can polymerize under acidic conditions, forming long-chain polymers. This is more likely to occur at higher temperatures and with high concentrations of the acid catalyst.
- Ether Formation: Alcohols can react with each other to form ethers under acidic conditions. In the case of 2-butanol, this can lead to the formation of di-sec-butyl ether.
Experimental Considerations
To perform the hydration of 2-butene in the laboratory, the following considerations are important:
- Safety: Concentrated acids, such as sulfuric acid, are corrosive and must be handled with care. Appropriate personal protective equipment (PPE), including gloves, goggles, and a lab coat, should be worn.
- Reaction Setup: The reaction is typically carried out in a round-bottom flask equipped with a magnetic stirrer, condenser, and heating mantle. The condenser is necessary to prevent the loss of volatile reactants and products.
- Reaction Monitoring: The progress of the reaction can be monitored using techniques such as gas chromatography (GC) or thin-layer chromatography (TLC).
- Workup: After the reaction is complete, the product is typically isolated by extraction with an organic solvent, followed by washing, drying, and distillation.
Alternative Methods for Alkene Hydration
Besides the traditional acid-catalyzed hydration, other methods can be used to hydrate alkenes:
- Oxymercuration-Demercuration: This method involves the reaction of the alkene with mercuric acetate (Hg(OAc)₂) in water, followed by reduction with sodium borohydride (NaBH₄). This method is highly regioselective and follows Markovnikov's rule.
- Hydroboration-Oxidation: This method involves the reaction of the alkene with borane (BH₃) or a borane derivative, followed by oxidation with hydrogen peroxide (H₂O₂) in the presence of a base. This method is anti-Markovnikov and syn-stereospecific.
Industrial Applications
The hydration of alkenes is an important industrial process for the production of alcohols. Alcohols are used as solvents, intermediates in the synthesis of other chemicals, and as fuel additives. For example, ethanol is produced by the hydration of ethene, and isopropanol is produced by the hydration of propene.
Key Concepts Revisited
To summarize, let's revisit the key concepts discussed:
- Alkene Hydration: The addition of water to an alkene to form an alcohol.
- Markovnikov's Rule: In the addition of a protic acid HX to an alkene, the hydrogen atom adds to the carbon atom with the greater number of hydrogen atoms, and the X group adds to the carbon atom with the fewer hydrogen atoms.
- Carbocation Intermediate: A positively charged carbon atom formed during the reaction.
- Stereochemistry: The spatial arrangement of atoms in a molecule, including the formation of enantiomers.
- Acid Catalyst: A substance that speeds up the reaction without being consumed in the process.
FAQ Section
Q: What is the product of the hydration of 2-butene?
A: The main product is 2-butanol, which is a racemic mixture of (R)-2-butanol and (S)-2-butanol.
Q: Why is an acid catalyst needed for the hydration of 2-butene?
A: The acid catalyst protonates the double bond, making it more susceptible to nucleophilic attack by water. Without the acid catalyst, the reaction would be very slow.
Q: What is Markovnikov's rule, and how does it apply to the hydration of 2-butene?
A: Markovnikov's rule states that in the addition of a protic acid HX to an alkene, the hydrogen atom adds to the carbon atom with the greater number of hydrogen atoms. In the case of 2-butene, both carbon atoms of the double bond are equally substituted, so the rule doesn't favor one product over another, leading to 2-butanol.
Q: Are there any side reactions that can occur during the hydration of 2-butene?
A: Yes, side reactions can include alkene isomerization, polymerization, and ether formation.
Q: Can the hydration of 2-butene produce different isomers of butanol?
A: Yes, the reaction produces 2-butanol. Since the second carbon becomes a chiral center, a racemic mixture of (R)-2-butanol and (S)-2-butanol is formed.
Q: How does temperature affect the hydration reaction?
A: Higher temperatures generally increase the reaction rate, but excessively high temperatures can lead to side reactions.
Conclusion
The hydration of 2-butene is a fundamental reaction in organic chemistry that produces 2-butanol. The reaction mechanism involves protonation, nucleophilic attack, and deprotonation, and it follows Markovnikov's rule. The product is a racemic mixture of (R)-2-butanol and (S)-2-butanol due to the formation of a chiral center during the reaction. Understanding this reaction is crucial for students and professionals in chemistry as it provides insight into alkene reactivity and the formation of alcohols. Factors such as acid concentration, temperature, and solvent can affect the reaction rate and yield. Additionally, alternative methods for alkene hydration, such as oxymercuration-demercuration and hydroboration-oxidation, offer different regiochemical and stereochemical outcomes.
Latest Posts
Latest Posts
-
Amoeba Sisters Asexual And Sexual Reproduction
Nov 28, 2025
-
Shallow Vs Deep Depth Of Field
Nov 28, 2025
-
Which Role Is Responsible For Defining The Detailed Database Design
Nov 28, 2025
-
Financial Statements Include Which Of The Following Two
Nov 28, 2025
-
Oracion A La Santa Muerte Para Pedir Un Favor Urgente
Nov 28, 2025
Related Post
Thank you for visiting our website which covers about Draw The Product Of The Hydration Of 2 Butene . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.