Balanced Equation For The Decomposition Of Hydrogen Peroxide
planetorganic
Dec 01, 2025 · 11 min read
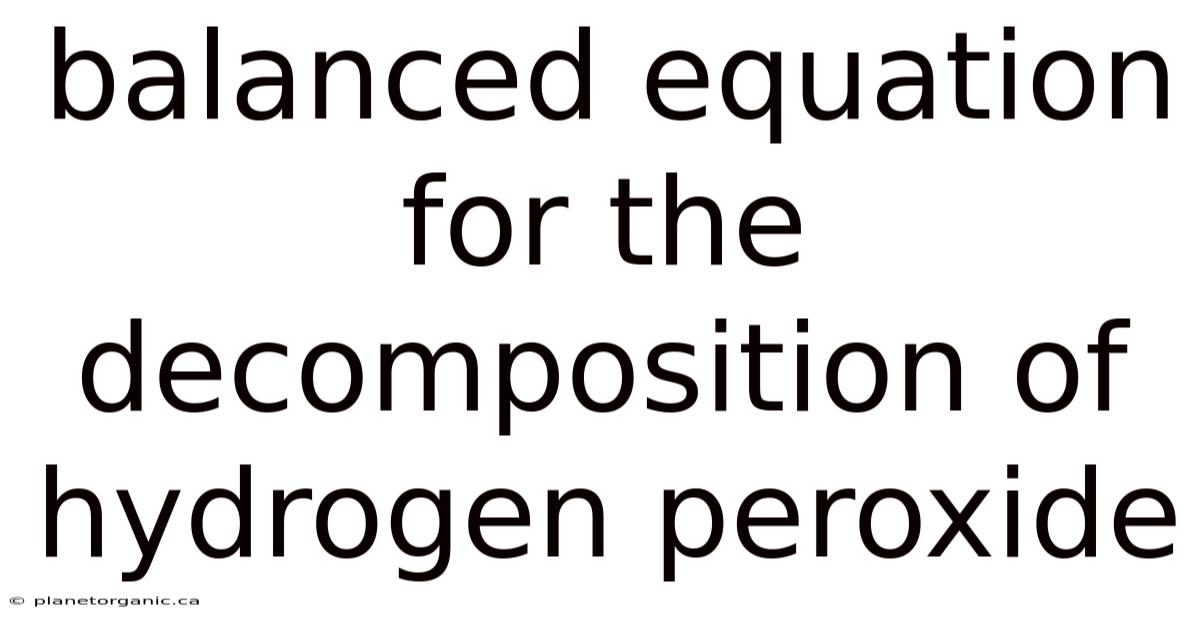
Table of Contents
The decomposition of hydrogen peroxide is a fascinating chemical reaction, one that demonstrates the principles of chemical kinetics and equilibrium. Understanding the balanced equation for this process is fundamental to grasping how hydrogen peroxide breaks down into water and oxygen, and how we can influence this decomposition.
Understanding Hydrogen Peroxide and Decomposition
Hydrogen peroxide (H₂O₂) is a chemical compound known for its oxidizing properties. In its pure form, it's a colorless liquid, but it's commonly found in diluted solutions for various applications, from bleaching and disinfection to rocket propulsion.
Decomposition, in chemical terms, refers to the breakdown of a compound into simpler substances. In the case of hydrogen peroxide, it decomposes into water (H₂O) and oxygen gas (O₂). This reaction is thermodynamically favorable, meaning it naturally tends to occur spontaneously. However, the rate at which it happens can be quite slow under normal conditions.
The Unbalanced Equation: A Starting Point
Before balancing any equation, we must first represent the reaction in its simplest, unbalanced form. For the decomposition of hydrogen peroxide, this looks like:
H₂O₂ → H₂O + O₂
This equation tells us what the reactants (on the left side of the arrow) and the products (on the right side) are. However, it doesn't tell us anything about the quantity of each molecule involved. It violates the fundamental law of conservation of mass, which dictates that matter cannot be created or destroyed in a chemical reaction. The number of atoms of each element must be the same on both sides of the equation.
Why Balancing is Necessary
Balancing chemical equations is not just a matter of making things look neat and tidy. It's about accurately representing the quantitative relationships between the substances involved in a chemical reaction. A balanced equation allows us to:
- Predict the amount of product formed: Knowing the exact ratio of reactants to products allows us to calculate how much of each product will be generated from a given amount of reactant.
- Understand stoichiometry: Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. Balanced equations are the foundation of stoichiometric calculations.
- Apply the law of conservation of mass: By ensuring the number of atoms of each element is the same on both sides of the equation, we uphold the fundamental principle of mass conservation.
- Design experiments and processes: In industrial and laboratory settings, balanced equations are crucial for designing efficient and safe chemical processes.
Steps to Balancing the Hydrogen Peroxide Decomposition Equation
Balancing chemical equations is a skill that requires a systematic approach. Here's a step-by-step guide to balancing the hydrogen peroxide decomposition equation:
-
Write the unbalanced equation: As mentioned earlier, our starting point is:
H₂O₂ → H₂O + O₂
-
Count the atoms of each element on both sides:
- Left side (Reactant):
- Hydrogen (H): 2
- Oxygen (O): 2
- Right side (Products):
- Hydrogen (H): 2
- Oxygen (O): 3
- Left side (Reactant):
-
Identify the element that is not balanced: In this case, oxygen is not balanced (2 on the left, 3 on the right).
-
Balance the element by adding coefficients: A coefficient is a number placed in front of a chemical formula to indicate the number of molecules of that substance. We need to find coefficients that will make the number of oxygen atoms equal on both sides.
-
A common strategy is to start by balancing the element that appears in the fewest number of formulas. In this case, oxygen appears in all three formulas.
-
To get an even number of oxygen atoms on the right side, we can start by placing a coefficient of "2" in front of the water molecule (H₂O):
H₂O₂ → 2H₂O + O₂
-
Now, recount the atoms:
- Left side (Reactant):
- Hydrogen (H): 2
- Oxygen (O): 2
- Right side (Products):
- Hydrogen (H): 4
- Oxygen (O): 4 (2 from 2*H₂O + 2 from O₂)
- Left side (Reactant):
-
Hydrogen is now unbalanced. To balance the hydrogen, we place a coefficient of "2" in front of the hydrogen peroxide molecule (H₂O₂):
2H₂O₂ → 2H₂O + O₂
-
Recount the atoms one last time:
- Left side (Reactant):
- Hydrogen (H): 4 (2 * 2)
- Oxygen (O): 4 (2 * 2)
- Right side (Products):
- Hydrogen (H): 4 (2 * 2)
- Oxygen (O): 4 (2 from 2*H₂O + 2 from O₂)
- Left side (Reactant):
-
-
Verify that the equation is balanced: The number of atoms of each element is now the same on both sides of the equation. The equation is balanced!
The Balanced Equation for Hydrogen Peroxide Decomposition
The balanced equation for the decomposition of hydrogen peroxide is:
2H₂O₂ → 2H₂O + O₂
This equation tells us that two molecules of hydrogen peroxide decompose to produce two molecules of water and one molecule of oxygen gas.
Factors Affecting the Rate of Decomposition
While the decomposition of hydrogen peroxide is spontaneous, its rate can be significantly influenced by several factors:
- Temperature: Higher temperatures generally accelerate the rate of decomposition. This is because increased temperature provides more energy for the molecules to overcome the activation energy barrier for the reaction.
- Catalysts: Catalysts are substances that speed up a chemical reaction without being consumed in the process. Several substances can catalyze the decomposition of hydrogen peroxide, including:
- Manganese dioxide (MnO₂): This is a common catalyst used in laboratory demonstrations.
- Potassium iodide (KI): Iodide ions act as catalysts in solution.
- Enzymes: Certain enzymes, such as catalase, are highly efficient catalysts for hydrogen peroxide decomposition in biological systems.
- Light: Exposure to light can also increase the rate of decomposition, especially ultraviolet (UV) light. This is why hydrogen peroxide is often stored in dark containers.
- pH: The pH of the solution can affect the stability of hydrogen peroxide. Decomposition is generally faster at higher pH values (more basic conditions).
- Contaminants: The presence of certain contaminants, such as metal ions, can also catalyze the decomposition.
The Role of Catalysts: A Closer Look
Catalysts play a critical role in accelerating the decomposition of hydrogen peroxide. They achieve this by providing an alternative reaction pathway with a lower activation energy. The activation energy is the minimum amount of energy required for a reaction to occur. By lowering this barrier, catalysts allow the reaction to proceed much faster.
For example, manganese dioxide (MnO₂) acts as a heterogeneous catalyst, meaning it exists in a different phase from the reactants (solid MnO₂ in a liquid H₂O₂ solution). The MnO₂ provides a surface on which the hydrogen peroxide molecules can adsorb and react more easily. The mechanism is complex, but involves changes in the oxidation state of the manganese.
Enzymes like catalase are biological catalysts that are incredibly efficient at decomposing hydrogen peroxide. Catalase contains an iron atom at its active site, which facilitates the breakdown of H₂O₂ into water and oxygen. The enzyme-substrate complex formed during the reaction drastically reduces the activation energy, allowing the reaction to proceed at astonishingly high rates.
Applications of Hydrogen Peroxide Decomposition
The decomposition of hydrogen peroxide has numerous applications in various fields:
- Rocket Propulsion: Concentrated hydrogen peroxide can be decomposed to generate high-pressure steam and oxygen, which can be used as a propellant in rockets.
- Bleaching: Hydrogen peroxide is a common bleaching agent used in the textile and paper industries. The decomposition releases oxygen, which oxidizes the colored compounds, making them colorless.
- Disinfection and Sterilization: Hydrogen peroxide is used as a disinfectant to kill bacteria, viruses, and fungi. The released oxygen acts as an oxidizing agent, damaging the cellular components of microorganisms.
- Wastewater Treatment: Hydrogen peroxide can be used to remove pollutants from wastewater. The decomposition generates hydroxyl radicals (OH), which are powerful oxidizing agents that can break down organic contaminants.
- Laboratory Demonstrations: The decomposition of hydrogen peroxide, especially when catalyzed by manganese dioxide or potassium iodide, is a popular demonstration in chemistry classes to illustrate chemical kinetics and catalysis. The rapid production of oxygen gas can create dramatic effects, such as the "elephant toothpaste" experiment.
- Medical Applications: Hydrogen peroxide is used in medicine as a mild antiseptic for cleaning wounds and as a mouthwash.
Safety Precautions
While hydrogen peroxide is widely used, it's important to handle it with care. Concentrated solutions can be corrosive and can cause burns to the skin and eyes.
- Wear appropriate personal protective equipment (PPE): This includes gloves, eye protection, and lab coats.
- Work in a well-ventilated area: Decomposition releases oxygen gas, which can build up in confined spaces.
- Avoid contact with skin and eyes: If contact occurs, rinse immediately with plenty of water.
- Store hydrogen peroxide in a cool, dark place: This helps to slow down the rate of decomposition.
- Never mix hydrogen peroxide with incompatible materials: Certain substances, such as strong reducing agents, can react violently with hydrogen peroxide.
The Scientific Explanation of the Decomposition
The decomposition of hydrogen peroxide is an exothermic reaction, meaning it releases heat. The overall reaction can be represented by the following thermochemical equation:
2H₂O₂(l) → 2H₂O(l) + O₂(g) ΔH = -196 kJ/mol
The negative value of ΔH (enthalpy change) indicates that the reaction releases energy in the form of heat. This energy release contributes to the spontaneity of the reaction.
The mechanism of the decomposition is complex and depends on the specific conditions, including the presence of catalysts. In the absence of catalysts, the reaction proceeds through a series of elementary steps involving the breaking and forming of chemical bonds. The rate-determining step is typically the homolytic cleavage of the O-O bond in hydrogen peroxide, which requires a significant amount of energy.
Catalysts lower the activation energy by providing an alternative reaction pathway. For example, in the presence of iodide ions (I⁻), the decomposition can proceed through the following mechanism:
- H₂O₂ (aq) + I⁻ (aq) → H₂O (l) + IO⁻ (aq)
- H₂O₂ (aq) + IO⁻ (aq) → H₂O (l) + O₂ (g) + I⁻ (aq)
In this mechanism, the iodide ion is first oxidized to hypoiodite ion (IO⁻), which then reacts with another molecule of hydrogen peroxide to produce oxygen gas and regenerate the iodide ion. The iodide ion acts as a catalyst because it is consumed in the first step but regenerated in the second step.
Common Mistakes to Avoid When Balancing Equations
Balancing chemical equations can be tricky, and it's easy to make mistakes. Here are some common errors to avoid:
- Changing Subscripts: Subscripts in chemical formulas indicate the number of atoms of each element in a molecule. Changing subscripts alters the identity of the substance. Only coefficients should be adjusted when balancing equations.
- Not Balancing All Elements: Make sure that the number of atoms of every element is the same on both sides of the equation.
- Forgetting to Distribute Coefficients: When a coefficient is placed in front of a formula containing multiple atoms, remember to multiply the coefficient by the subscript of each atom. For example, in 2H₂O, there are 4 hydrogen atoms (2 * 2) and 2 oxygen atoms (2 * 1).
- Not Simplifying Coefficients: After balancing the equation, check if the coefficients can be simplified by dividing them by a common factor. The coefficients should be in the simplest whole-number ratio.
- Assuming Diatomic Elements: Remember that certain elements, such as hydrogen, oxygen, nitrogen, and the halogens, exist as diatomic molecules (H₂, O₂, N₂, F₂, Cl₂, Br₂, I₂) in their elemental form. Make sure to account for this when balancing equations involving these elements.
FAQ About Hydrogen Peroxide Decomposition
- Is the decomposition of hydrogen peroxide reversible?
- No, the decomposition of hydrogen peroxide is essentially irreversible under normal conditions. The products, water and oxygen, do not readily recombine to form hydrogen peroxide.
- What is the shelf life of hydrogen peroxide?
- The shelf life of hydrogen peroxide depends on the concentration and storage conditions. Diluted solutions (3%) typically have a shelf life of several months if stored properly in a cool, dark place. Concentrated solutions are more stable but can still decompose over time.
- Can hydrogen peroxide explode?
- Concentrated hydrogen peroxide (above 70%) can be explosive under certain conditions, such as when heated or mixed with incompatible materials. However, diluted solutions (3-6%) commonly used for household purposes are not explosive.
- Why is hydrogen peroxide stored in brown bottles?
- Hydrogen peroxide is stored in brown or opaque bottles to protect it from light, which can accelerate its decomposition.
- Is hydrogen peroxide environmentally friendly?
- Yes, hydrogen peroxide is considered to be an environmentally friendly chemical because it decomposes into water and oxygen, which are both harmless to the environment.
Conclusion
Understanding the balanced equation for the decomposition of hydrogen peroxide is crucial for comprehending the stoichiometry, kinetics, and applications of this important chemical reaction. The balanced equation 2H₂O₂ → 2H₂O + O₂ provides a quantitative representation of the reaction, allowing us to predict the amount of products formed from a given amount of reactant. The rate of decomposition can be influenced by various factors, including temperature, catalysts, light, and pH. Hydrogen peroxide has numerous applications in various fields, from rocket propulsion and bleaching to disinfection and wastewater treatment. By following safe handling practices and understanding the underlying principles, we can effectively utilize the properties of hydrogen peroxide and harness its benefits in a variety of applications. The beauty of chemistry lies in the understanding and manipulation of these fundamental reactions, and the decomposition of hydrogen peroxide serves as a compelling example of the power and elegance of chemical principles.
Latest Posts
Latest Posts
-
Which Action Is An Administrative Safeguard
Dec 01, 2025
-
Efapel Centro P Inter Rotativo 90762tdu
Dec 01, 2025
-
5 Ft 7 In Inches In Meters
Dec 01, 2025
-
What Is The Difference Between A Parameter And A Statistic
Dec 01, 2025
-
Which Group Is An Example Of A Commodity Organization
Dec 01, 2025
Related Post
Thank you for visiting our website which covers about Balanced Equation For The Decomposition Of Hydrogen Peroxide . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.