A Hydrate Of Cocl2 With A Mass Of 6.00g
planetorganic
Dec 03, 2025 · 10 min read
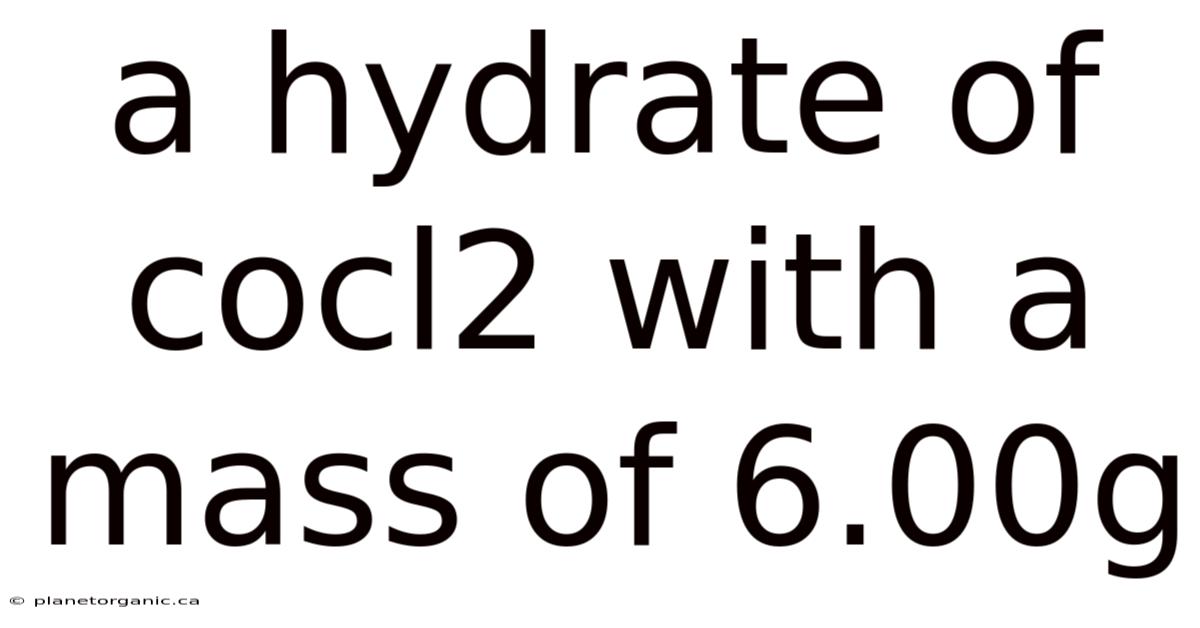
Table of Contents
Cobalt chloride hexahydrate, often abbreviated as CoCl₂·6H₂O, is a captivating inorganic compound renowned for its vibrant color changes based on hydration levels. This article delves into the fascinating world of cobalt chloride hexahydrate, exploring its properties, uses, and how to calculate its composition when you have a 6.00g sample.
Understanding Cobalt Chloride Hexahydrate (CoCl₂·6H₂O)
Cobalt chloride hexahydrate is a chemical compound comprised of cobalt (Co), chlorine (Cl), and water (H₂O) molecules arranged in a specific crystal lattice. The term "hexahydrate" signifies that each cobalt chloride molecule is associated with six water molecules. Its most distinctive feature is its ability to change color depending on its state of hydration. When fully hydrated, it exhibits a deep, rich pink or magenta color. As it loses water molecules, typically through heating or exposure to a desiccating environment, it transitions to a violet-blue hue. This color change makes it a useful indicator of humidity and a popular component in various scientific demonstrations.
Properties of CoCl₂·6H₂O
- Chemical Formula: CoCl₂·6H₂O
- Molar Mass: 237.93 g/mol (This is the sum of the atomic masses of one cobalt atom, two chlorine atoms, and six water molecules)
- Appearance: Typically appears as reddish-purple or magenta crystals when fully hydrated.
- Solubility: Highly soluble in water, alcohol, and acetone.
- Hygroscopic: Readily absorbs moisture from the air. This is the reason it's often used as a humidity indicator.
- Color Change: Pink (hydrated) to violet/blue (dehydrated). This is its defining characteristic.
- Toxicity: Moderately toxic; avoid ingestion and prolonged skin contact. Always handle with appropriate safety precautions.
Uses of Cobalt Chloride Hexahydrate
The unique properties of cobalt chloride hexahydrate lead to a variety of applications:
- Humidity Indicator: Perhaps its most well-known use. Impregnated paper or silica gel containing CoCl₂·6H₂O changes color to indicate humidity levels.
- Invisible Ink: Dilute solutions of cobalt chloride hexahydrate can be used as invisible ink. The writing becomes visible when heated.
- Weather Forecasting Instruments: Used in some weather instruments to indicate humidity levels.
- Preparation of Other Cobalt Compounds: A common precursor for synthesizing other cobalt-containing compounds.
- Vitamin B12 Analogues: Used in the production of certain vitamin B12 analogues.
- Electroplating: Used in some electroplating processes.
- Veterinary Medicine: Historically used (though less common now) as a source of cobalt for livestock.
Analyzing a 6.00g Sample of CoCl₂·6H₂O: Calculations and Composition
Let's address the core of the prompt: analyzing a 6.00g sample of cobalt chloride hexahydrate. The goal here is to determine the number of moles of CoCl₂·6H₂O present in the sample and to calculate the mass percentage of each element (Co, Cl, H, and O) within the compound.
Step 1: Calculate the Number of Moles of CoCl₂·6H₂O
To determine the number of moles in the 6.00g sample, we'll use the following formula:
Moles = Mass / Molar Mass
We already know:
- Mass of CoCl₂·6H₂O sample = 6.00 g
- Molar Mass of CoCl₂·6H₂O = 237.93 g/mol
Therefore:
Moles of CoCl₂·6H₂O = 6.00 g / 237.93 g/mol = 0.0252 moles (approximately)
This means our 6.00g sample contains approximately 0.0252 moles of cobalt chloride hexahydrate.
Step 2: Calculating the Mass of Each Element in One Mole of CoCl₂·6H₂O
To determine the mass percentage of each element, we first need to calculate the mass of each element present in one mole of CoCl₂·6H₂O. We will use the atomic masses of each element:
- Cobalt (Co): 58.93 g/mol
- Chlorine (Cl): 35.45 g/mol
- Hydrogen (H): 1.01 g/mol
- Oxygen (O): 16.00 g/mol
Now, let's calculate the mass of each element in one mole of CoCl₂·6H₂O:
- Mass of Cobalt (Co): 1 Co atom/molecule * 58.93 g/mol = 58.93 g/mol
- Mass of Chlorine (Cl): 2 Cl atoms/molecule * 35.45 g/mol = 70.90 g/mol
- Mass of Hydrogen (H): 6 H₂O molecules/molecule * 2 H atoms/molecule * 1.01 g/mol = 12.12 g/mol
- Mass of Oxygen (O): 6 H₂O molecules/molecule * 1 O atom/molecule * 16.00 g/mol = 96.00 g/mol
Step 3: Calculating the Mass Percentage of Each Element in CoCl₂·6H₂O
Now we can calculate the mass percentage of each element using the following formula:
Mass Percentage = (Mass of Element in One Mole / Molar Mass of Compound) * 100%
- Mass Percentage of Cobalt (Co): (58.93 g/mol / 237.93 g/mol) * 100% = 24.77% (approximately)
- Mass Percentage of Chlorine (Cl): (70.90 g/mol / 237.93 g/mol) * 100% = 29.80% (approximately)
- Mass Percentage of Hydrogen (H): (12.12 g/mol / 237.93 g/mol) * 100% = 5.09% (approximately)
- Mass Percentage of Oxygen (O): (96.00 g/mol / 237.93 g/mol) * 100% = 40.35% (approximately)
Therefore, the mass percentages of each element in cobalt chloride hexahydrate are approximately:
- Cobalt (Co): 24.77%
- Chlorine (Cl): 29.80%
- Hydrogen (H): 5.09%
- Oxygen (O): 40.35%
It's crucial to note that these percentages apply to the compound cobalt chloride hexahydrate (CoCl₂·6H₂O) specifically. If the sample is not pure or has lost some of its water of hydration, these percentages will not be accurate.
Step 4: Calculating the Mass of Each Element in the 6.00g Sample
Finally, we can calculate the mass of each element present in our original 6.00g sample. To do this, we use the mass percentages calculated above:
Mass of Element in Sample = (Mass Percentage of Element / 100%) * Mass of Sample
- Mass of Cobalt (Co) in 6.00g sample: (24.77% / 100%) * 6.00 g = 1.49 g (approximately)
- Mass of Chlorine (Cl) in 6.00g sample: (29.80% / 100%) * 6.00 g = 1.79 g (approximately)
- Mass of Hydrogen (H) in 6.00g sample: (5.09% / 100%) * 6.00 g = 0.305 g (approximately)
- Mass of Oxygen (O) in 6.00g sample: (40.35% / 100%) * 6.00 g = 2.42 g (approximately)
So, in our 6.00g sample of cobalt chloride hexahydrate, we have approximately:
- 1.49 g of Cobalt
- 1.79 g of Chlorine
- 0.305 g of Hydrogen
- 2.42 g of Oxygen
You can verify that the sum of these masses (1.49 + 1.79 + 0.305 + 2.42 = 6.005) is very close to the original sample mass of 6.00g, accounting for rounding errors.
Hydration and Dehydration of Cobalt Chloride Hexahydrate: A Deeper Dive
The color change exhibited by cobalt chloride hexahydrate is directly related to the presence and absence of water molecules coordinated to the cobalt ion. This process is reversible and can be represented by the following equilibrium:
- 6H₂O
-
Hydration (Pink): When cobalt chloride is hydrated, the cobalt ion (Co²⁺) is surrounded by six water molecules, forming a complex ion [Co(H₂O)₆]²⁺. This complex absorbs light in a particular region of the visible spectrum, resulting in the characteristic pink color. The chloride ions (Cl⁻) are present as counterions.
-
Dehydration (Blue): When heated or placed in a dry environment, the equilibrium shifts to the right. The water molecules are removed from the cobalt ion, and the chloride ions move in to coordinate directly with the cobalt ion, forming a tetrahedral complex [CoCl₄]²⁻. This different coordination environment alters the electronic structure of the cobalt ion, causing it to absorb light in a different region of the spectrum, resulting in the blue color.
The degree of color change is proportional to the amount of water lost or gained. Partial dehydration can result in intermediate colors, such as violet.
Factors Affecting Hydration/Dehydration
Several factors can influence the hydration state of cobalt chloride hexahydrate:
- Temperature: Higher temperatures favor dehydration, as heat provides the energy needed to break the bonds between the cobalt ion and water molecules.
- Humidity: Low humidity favors dehydration, as the dry air draws water molecules away from the cobalt chloride. High humidity favors hydration, as the air provides a source of water molecules to coordinate with the cobalt ion.
- Presence of Desiccants: Desiccants (drying agents) such as silica gel or calcium chloride can effectively remove water from the environment, promoting dehydration of the cobalt chloride.
- Partial Pressure of Water: The partial pressure of water in the surrounding atmosphere also plays a role. Lower partial pressure of water favors dehydration.
Practical Implications of Hydration/Dehydration
The reversible hydration/dehydration of cobalt chloride has several practical implications:
- Humidity Sensors: Cobalt chloride-impregnated paper or silica gel is used in humidity sensors and indicators. The color change provides a visual indication of the relative humidity.
- Desiccants: Cobalt chloride can be added to desiccants to indicate their effectiveness. When the desiccant is saturated with water, the cobalt chloride will turn pink, indicating that the desiccant needs to be replaced or regenerated.
- Experiments: The color change can be used in educational demonstrations to illustrate the principles of chemical equilibrium, hydration, and dehydration.
- Artwork: Artists have used cobalt chloride's color-changing property to create reactive artwork that changes appearance with humidity.
Safety Precautions When Handling Cobalt Chloride Hexahydrate
While cobalt chloride hexahydrate has many useful applications, it's crucial to handle it with care due to its potential toxicity:
- Toxicity: Cobalt chloride is considered moderately toxic. Ingestion can cause nausea, vomiting, and diarrhea. Prolonged exposure can lead to more serious health problems.
- Skin and Eye Irritation: Cobalt chloride can cause skin and eye irritation. Avoid contact with skin and eyes.
- Inhalation: Avoid inhaling cobalt chloride dust or fumes.
- Personal Protective Equipment (PPE): Wear appropriate PPE, including gloves, safety glasses, and a lab coat, when handling cobalt chloride.
- Ventilation: Work in a well-ventilated area to minimize inhalation of dust or fumes.
- Storage: Store cobalt chloride in a tightly closed container in a cool, dry place. Keep away from incompatible materials.
- Disposal: Dispose of cobalt chloride waste properly according to local regulations. Do not pour it down the drain.
Common Questions about Cobalt Chloride Hexahydrate (FAQ)
- What is the difference between cobalt chloride and cobalt chloride hexahydrate? Cobalt chloride (CoCl₂) refers to the anhydrous form of the compound, while cobalt chloride hexahydrate (CoCl₂·6H₂O) is the hydrated form containing six water molecules per cobalt chloride molecule. They have different properties, including color and molar mass.
- Why does cobalt chloride change color? The color change is due to the change in the coordination environment around the cobalt ion (Co²⁺) as it gains or loses water molecules. This changes the way the cobalt ion absorbs light.
- Is cobalt chloride hexahydrate safe? It is moderately toxic and should be handled with care. Avoid ingestion, skin contact, and inhalation. Wear appropriate PPE when handling it.
- How can I dehydrate cobalt chloride hexahydrate? Heating the compound is the most common method. You can also place it in a desiccator with a drying agent.
- How can I rehydrate dehydrated cobalt chloride? Expose it to humid air or add water to it.
- Can I use cobalt chloride to test for water? Yes, the color change from blue to pink indicates the presence of water.
- Where can I buy cobalt chloride hexahydrate? It can be purchased from chemical suppliers and online retailers that sell laboratory chemicals.
- What is the molar mass of anhydrous cobalt chloride (CoCl₂)? The molar mass of CoCl₂ is 129.84 g/mol. This is significantly different from the molar mass of the hexahydrate (237.93 g/mol).
- Does cobalt chloride hexahydrate dissolve in nonpolar solvents? While it readily dissolves in polar solvents like water, alcohol, and acetone, its solubility in nonpolar solvents is limited.
Conclusion
Cobalt chloride hexahydrate is a fascinating chemical compound with diverse applications stemming from its unique ability to change color depending on its hydration state. Understanding its properties, uses, and safety precautions is crucial for anyone working with this compound. By following the steps outlined in this article, you can accurately calculate the composition of a cobalt chloride hexahydrate sample and appreciate the chemistry behind its intriguing color changes. Remember to always handle chemicals with respect and adhere to safety guidelines to ensure a safe and productive experience. The calculations presented allow for a quantitative understanding of the elemental composition of a given sample, illustrating the fundamental principles of stoichiometry and chemical analysis. The reversible hydration/dehydration process highlights important concepts in chemical equilibrium and the impact of environmental factors on chemical systems.
Latest Posts
Latest Posts
-
Which Region Of The Vertebral Column Contains The Smallest Vertebrae
Dec 03, 2025
-
Wordly Wise Book 7 Lesson 12
Dec 03, 2025
-
Applying Sunscreen Will Help Olivia Defend Against Getting
Dec 03, 2025
-
What Is The Difference Between Natural Selection And Selective Breeding
Dec 03, 2025
-
Which Of The Following Statements About Enzymes Are True
Dec 03, 2025
Related Post
Thank you for visiting our website which covers about A Hydrate Of Cocl2 With A Mass Of 6.00g . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.